As the saying goes, “Don’t judge a book by its cover” but with Listeria, the opposite is true. Its ‘cover’ is exactly what makes it dangerous.
Most food safety teams know where Listeria likes to live, be it the drains, the slicers, the cold rooms or the damp corners that never fully dry. But more than chasing locations of Listeria monocytogenes on a map, it requires understanding the surface biology of the organism itself. The outer cover that helps it latch onto equipment, resist sanitizers, and build biofilms that stay rooted for months.
This outer layer has a name: the surfactome. And the more we understand it, the smarter our sanitation and validation programs can become.
The Real Challenge: Instead of Just "Sitting" on Surfaces, Listeria Grips Them
Listeria is a cold-loving survivor that can grow where other pathogens fail. But the real danger isn’t its ability to survive, it’s its ability to stick, spread, and persist.
Here’s what makes up this grip-enhancing outer layer:
| Surfactome Component | Function | Why It Matters |
|---|---|---|
| Peptidoglycan | Structural support | Helps Listeria withstand cleaning pressure. |
| Teichoic acids | Surface charge & adhesion | Improves attachment to steel, plastic, rubber. |
| Surface proteins | Hooks, anchors, enzymes | Initiates early attachment and biofilm formation. |

A schematic diagram of the Listeria monocytogenes surfactome
Reference: https://pmc.ncbi.nlm.nih.gov/articles/PMC8313260/figure/mbt213847-fig-0001/
Together, they constitute the surfactome, Listeria’s adhesive personality. Once it latches on, the transformation is rapid and inexorable: from solitary cells to an entrenched biofilm.
And these biofilms are why Listeria remains one of the most persistent and disruptive pathogens in food processing environments.
Biofilms: The Long-Term Survival Shelter of Listeria
A Listeria biofilm is no casual congregation. It is an organised, fortified settlement, sheathed in DNA, proteins, and polysaccharides that renders conventional sanitation almost laughably ineffective.
Biofilms form in three steps:

Phases of the formation and development of biofilms.
Reference: https://medcraveonline.com/JMEN/JMEN-01-00014.pdf
Step 1: Initial adhesion → Listeria attaches using its surfactome
Step 2: Microcolony formation → cells start multiplying
Step 3: Mature biofilm → a fully fortified community forms
Once a biofilm matures, typical sanitation steps may reduce surface counts but rarely eliminate the entire community. This is why some facilities see the same Listeria strains returning repeatedly in environmental monitoring as those strains have built a “home.”
Why Biofilms Are So Dangerous
| Biofilm Characteristics | Impact on Plant |
|---|---|
| Chemical resistance | Sanitizers lose effectiveness |
| Physical tolerance | Pressure doesn’t remove cells |
| Long-term persistence | Same strain returns repeatedly |
| Cell shedding | Downstream contamination risk |
Why L. innocua Matters in Biofilm Research
In cleaning validations, processors often use L. innocua, a harmless relative that mirrors the pathogenic species in almost every behavioural aspect except virulence.
The difference is that L. monocytogenes carries virulence factors that make it dangerous, especially for RTE (ready-to-eat) environments. Understanding how surfactomes differ between the two species helps food facilities evaluate cleaning tools safely while still targeting authentic Listeria behavior.
For processors, this brings practical advantages:
- Safe on-floor testing: Teams can evaluate detergents, enzymes, and mechanical cleaning directly on equipment without introducing a pathogen.
- Realistic biofilm challenges: L. innocua forms robust biofilms, helping plants test how well their sanitation steps break down tough residues.
- Training without risk: Sanitation teams can practice correct cleaning techniques using an organism that behaves like Listeria.
- Cost-effective validation: Multiple trials can be run easily and affordably compared to pathogen-based lab studies.
Better audit readiness:
Facilities generate proof that their cleaning programs work against Listeria-like organisms.
What This Means for Food Processing Facilities
If Listeria’s survival advantage comes from its surface biology, then effective control needs to focus on:
1. Prioritise Disruption Over Disinfection
Sanitizers alone do poorly against biofilms. Before killing, you must expose the cells.
A strong sanitation approach must include:
- Detergents that dismantle the biofilm matrix
- Enzymatic cleaners for drains and equipment undersides
- Mechanical force like scrubbing, agitation, brushing
- Optimised water flow and temperature
2. Re-examine High-Risk Zones
Listeria doesn’t randomly choose its hiding spots. It follows moisture.
High-risk surfaces include:
- Drains and floor-wall junctions
- Pitted or scratched metal
- Conveyor undersides and hollow rollers
- Gaskets, seals, door tracks, valves
- Cold, humid corners reluctant to dry
3. Understanding strain behavior
Not all Listeria strains form biofilms equally. Some strains are strong biofilm formers and become persistent residents. Others attach weakly and disappear.
This is why:
- Facilities should track recurring strains
- Environmental monitoring should identify long-term patterns
- Surrogate testing must be paired with real-world validation
4. Building surfactome-aware sanitation programs
Since adhesion is driven by surface molecules, sanitation must be designed to:
- Break adhesion forces
- Remove the outer protective layers
- Target wall structures using enzymatic or oxidative chemistries
- Validate not only “sanitizer kill,” but biofilm removal
Practical Solutions for Food Processing Units
Below are actionable, operations-ready solutions grounded in what we know about Listeria’s surface biology:
Solution 1: Biofilm-focused cleaning cycles
- Add routine biofilm-disruption steps to the sanitation schedule
- Rotate cleaning chemistries to break down multiple matrix components
- Introduce enzyme-based cleaners for drains, conveyors, and cold rooms
Solution 2: Strengthen environmental monitoring
- Increase frequency in cold, wet, or high-traffic areas
- Use zone 3 & 4 data to predict zone 1 risks
- Conduct follow-up sampling 24–48 hours post-cleaning to detect regrowth
Solution 3: Repair or replace “biofilm-favored” equipment
Surfactome-driven adhesion increases on:
- Scratched surfaces
- Pitted metal
- Worn gaskets
- Hard-to-clean assemblies
Smooth, well-maintained equipment reduces Listeria’s ability to anchor.
Solution 4: Integrate dry-zone thinking
Where possible:
- Reduce standing water
- Improve air flow
- Fix slope issues leading to water pooling
- Add rapid-dry protocols after washdowns
Solution 5: Validate with surrogate organisms like L. innocua
- Realistic validation
- Safe assessment of cleaning protocols
- Repeatable testing
Better understanding of strain-to-strain differences
Benefits and Limitations of Surfacing-Based Listeria Control Strategies
✔ Benefits
- Targets the root cause of persistence
- Enhances long-term sanitation success
- Reduces contamination and recalls
- Improves traceability and regulatory confidence
✘ Limitations
- Requires structured validation
- May increase upfront investment
- Needs cross-department coordination
- Demands continuous monitoring and training
Still, the balance overwhelmingly favors adopting surfactome-aware strategies because the cost of ignoring biofilms is far greater than the cost of preventing them.
From Surface Awareness to Strategic Expertise
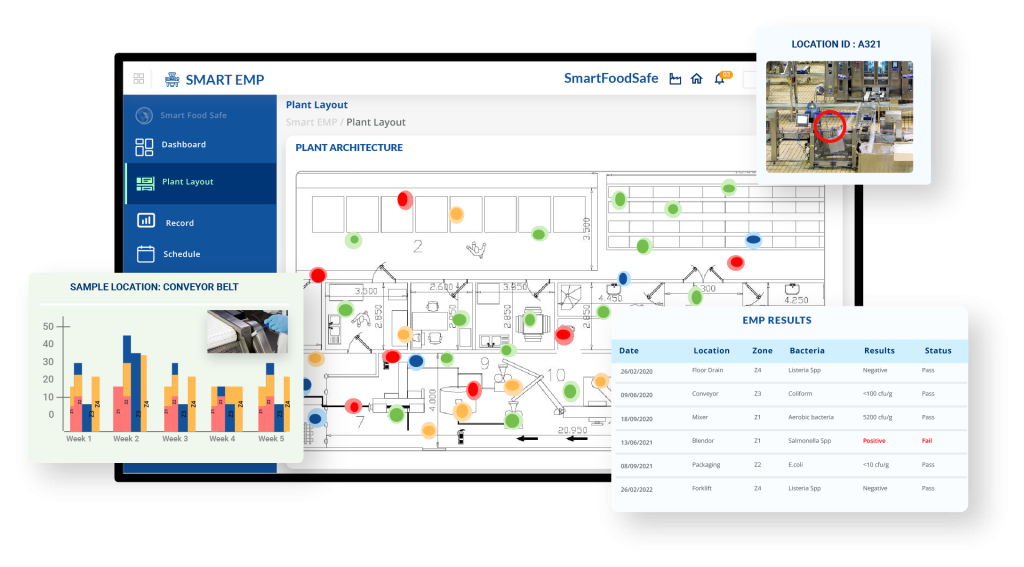
When processors reorient their strategies around this biological reality, focusing on adhesion, surfactome behaviour, and the mechanisms of early biofilm formation, they elevate their programs from routine compliance to true microbial management. Cleaning becomes more deliberate, environmental data becomes more revealing, and persistent strains lose their long-held advantage.
It is within this modern paradigm that platforms like Smart EMP demonstrate their quiet yet transformative significance. By discerning subtle environmental trends, detecting recurrent microbial signatures, and illuminating areas where biofilm pressure is emerging, Smart EMP equips facilities with a level of operational intelligence previously out of reach. It strengthens decision-making, sharpens prioritisation, and enables interventions at a moment when they are most effective before contamination becomes entrenched.
In essence, the future of Listeria control belongs to those who shift from chasing the pathogen to understanding and anticipating its behaviour. Facilities that combine surfactome-aware sanitation with intelligent, data-driven monitoring systems will stand not only better protected, but fundamentally more resilient. Such plants move confidently from reactive firefighting to proactive, science-led stewardship, a standard that defines the next generation of food safety.