An Environmental Monitoring Program (EMP) is vital for enabling manufacturers to detect, control, and prevent pathogens from lurking in their facilities. But how do you design an EMP that actually works? Which pathogens should be on your radar? And what’s the game plan if you get a positive test result? Below, we explore key questions and provide comprehensive answers to guide your EMP implementation.
Necessity of an EMP for your Facility
⇒ What is an Environmental Monitoring Program (EMP)?
An Environmental Monitoring Program (EMP) is a structured, written plan designed to monitor and test for pathogens, indicator organisms, or index organisms in food processing environments. Indicator organisms help assess the overall hygienic quality of the facility, while index organisms signal the potential presence of pathogens that thrive under similar conditions.
EMP plays a critical role in identifying microbial risks and ensuring food safety by evaluating how production processes and sanitation measures impact environmental hazards. This document aims to address common questions about EMPs and guide food producers in developing and implementing an effective monitoring program.
⇒ Why Does My Facility Need an EMP?
Under the Preventive Controls for Human Food (PCHF) regulation, facilities producing ready-to-eat (RTE) foods must implement environmental monitoring when an identified environmental hazard requires preventive control. RTE foods, such as salads, cooked meats, smoked fish, cheese, spices, and fresh produce, are not typically cooked before consumption. This makes it essential to minimize the risk of contamination from harmful pathogens.
An EMP serves as a verification tool to assess the effectiveness of cleaning and sanitation measures in controlling environmental hazards. However, before implementing an EMP, it is crucial to establish a robust sanitation program. A comprehensive sanitation plan includes validated cleaning and sanitizing procedures, employee training, written Standard Operating Procedures (SOPs), and thorough recordkeeping. Your EMP acts as a verification step to ensure these sanitation efforts effectively reduce microbial risks in your facility.
⇒ What Are the Consequences of Failing to Maintain an Effective EMP?
- Foodborne Illness: An ineffective EMP can lead to foodborne illness by failing to properly control contaminants, hygiene practices, and sanitation processes in the facility.
- Product Recalls: Contaminated food reaching consumers can severely impact brand reputation and financial stability.
- Regulatory Non-Compliance Penalties: These can result in legal actions and financial fines.
- Brand Reputation: A foodborne illness outbreak linked to the facility can cause long-term damage to business credibility.
Establishing a Strong EMP Framework
⇒ What are the essential elements of an effective EMP?
An EMP should be tailored to meet the specific needs of a facility, but certain key components are essential for an effective program.
- EMP Team – A cross-functional team should be assembled, including members with expertise in food safety, quality assurance, maintenance, and production. Their collective knowledge ensures comprehensive monitoring and swift issue resolution.
- Facility Map – A detailed map and floor plans should be included, clearly marking hygienic zones to help identify potential risk areas for sampling and testing.
- Process Flow – The EMP should document the entire production process, outlining each step from raw materials to finished products. This helps pinpoint critical areas where contamination risks may arise.
- Key Parameters – Clearly define testing requirements, including target organisms, sampling locations, testing methods, sampling frequency, number of samples collected per session, and the designated testing laboratory.
- Corrective Action Procedures – A well-defined corrective action plan should be in place to address any deviations from acceptable limits. This ensures that any detected risks are promptly managed and prevented from recurring.
- Records Management – Comprehensive documentation should be maintained, including training records, Standard Operating Procedures (SOPs), test results, and corrective action reports. Proper record-keeping supports compliance and continuous improvement.
By integrating these components, an EMP can effectively monitor environmental conditions, enhance food safety, and ensure regulatory compliance.
Developing a Targeted Sampling Plan with a Strategic Selection of Sampling Sites
⇒ How should sampling be distributed across facility zones?
Selecting appropriate sampling sites in your facility is critical for identifying potential contamination risks rather than simply confirming cleanliness. The goal should be to target areas where issues are most likely to arise rather than locations that are easy to clean and consistently meet acceptable limits. By focusing on high-risk areas, your EMP can better detect and mitigate contamination threats in the processing environment.
Understanding Sampling Zones
Environmental monitoring programs categorize facility areas into four sampling zones:
- Zone 1 – Surfaces that have direct contact with food.
- Zone 2 – Areas near food contact surfaces, such as equipment frameworks and control panels.
- Zone 3 – Non-food-contact areas within the processing environment, like floors, drains, and walls.
- Zone 4 – Areas outside the processing environment that could indirectly introduce contamination, including hallways and employee break rooms.
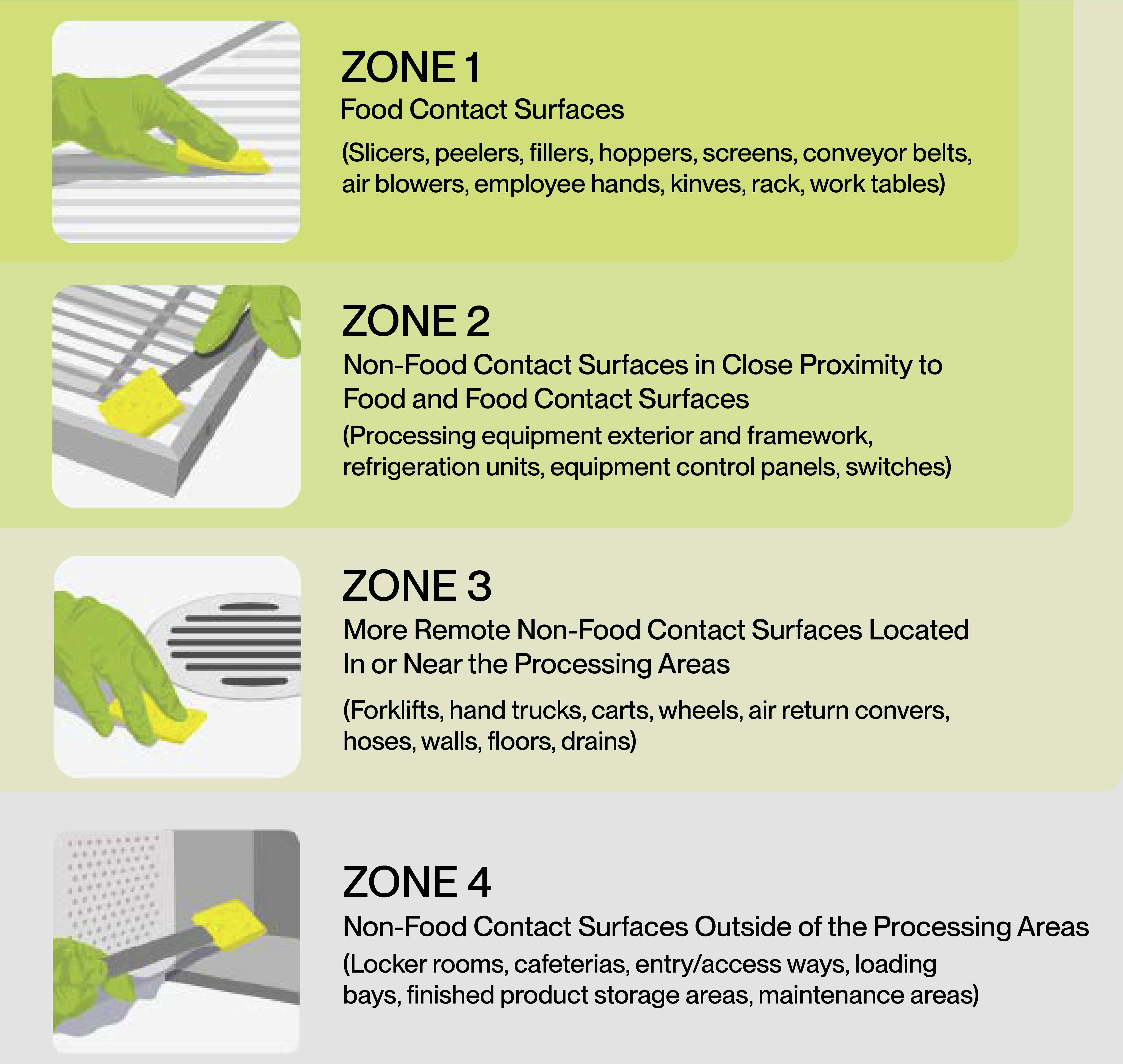
Reference: https://www.pubs.ext.vt.edu/content/dam/pubs_ext_vt_edu/FST/fst-445/FST-445.pdf
Best practices emphasize focusing on a mix of Zones 2 to 4 to monitor contamination risks while also periodically assessing Zone 1 for potential exposure.
Developing a Master Sampling List
A well-structured EMP includes a master list of potential sampling sites, prioritizing areas with the highest contamination risks. These should include:
- Hard-to-clean areas such as cracks, crevices, and corners.
- High-traffic zones where people, waste, or equipment frequently move.
- Locations where water or debris accumulates, increasing the likelihood of microbial growth.
- Post-kill step areas, where contamination risks can re-emerge.
- Sites with a history of positive pathogen results or previous non-compliance.
⇒ How do you determine sample locations and frequency?
While a facility might have hundreds of possible sampling sites, only a subset should be tested during each sampling event. The number of sites sampled depends on factors like facility size, production lines, and operational risks. To ensure a dynamic and effective monitoring program, it is essential to:
- Rotate sampling locations rather than consistently testing the same low-risk sites.
- Randomly select high-risk sites during each sampling event.
- Regularly assess new or emerging risks, such as pooled water or newly developed cracks in floors and walls.
By strategically selecting sampling sites, an EMP can provide actionable insights into the hygiene status of a facility, enabling proactive food safety management and regulatory compliance.
Identifying & Detecting Pathogens
⇒ What should an EMP focus on?
The specific targets of your Environmental Monitoring Program (EMP) depend on its objectives. Common microbial targets include ATP, spoilage organisms, indicator organisms, and foodborne pathogens. Additionally, EMPs may also test for allergens in facilities handling allergen-containing and non-allergen-containing products.
The table below outlines key target organisms and the purpose of testing each:
| Target | Purpose |
|---|---|
| ATP | ATP testing provides a quick and effective way to assess the hygienic condition of surfaces in your processing facility. |
| Spoilage Organisms | Used to verify sanitation effectiveness in the processing environment. Common examples include yeasts and molds. |
| Indicator Organisms | Helps assess the overall hygiene of the facility, understand microbial ecology, verify sanitation practices, and evaluate post-processing contamination risks. Common indicator organisms include total coliforms, fecal coliforms, and Enterococci. |
| Foodborne Pathogens | Identifies and eliminates potential environmental sources of pathogens such as Salmonella and Listeria monocytogenes. |
| Allergens | Ensures that allergen residues are not present on equipment or within the facility to prevent cross-contamination, particularly in facilities processing both allergen-containing and non-allergen-containing products. |
By selecting the appropriate targets, your EMP can help maintain a high level of food safety and hygiene in your facility.
⇒ How can false positives and negatives be minimized?
- Use validated testing methods tailored to facility conditions.
- Maintain strict sample-handling procedures to prevent cross-contamination.
- Train staff in correct swabbing techniques and ensure proper equipment calibration.
- Implement control samples to verify test accuracy.
Responding to Contamination Events
⇒ What steps should be taken if a test result is positive?
If a test result indicates contamination—either through a positive pathogen detection or microbial counts exceeding acceptable limits—it is crucial to take immediate corrective action as outlined in your EMP. Every non-compliant result should trigger a structured response to eliminate the contamination source and prevent recurrence.
Key Corrective Actions
The appropriate corrective measures will depend on the severity and nature of the issue but may include:
- Re-cleaning and sanitizing the affected area or equipment.
- Re-testing to confirm that corrective actions were effective.
- Repairing or replacing equipment if structural defects contribute to contamination.
- Holding or discarding affected products to prevent the distribution of unsafe food.
- Issuing a product recall, if necessary, to mitigate risks to consumers.
Conducting a root cause analysis is essential to identify the source of contamination. This may involve increased sampling in and around the affected area to pinpoint problem spots and prevent future occurrences. Proactively addressing contamination risks strengthens food safety measures and ensures compliance with regulatory standards.
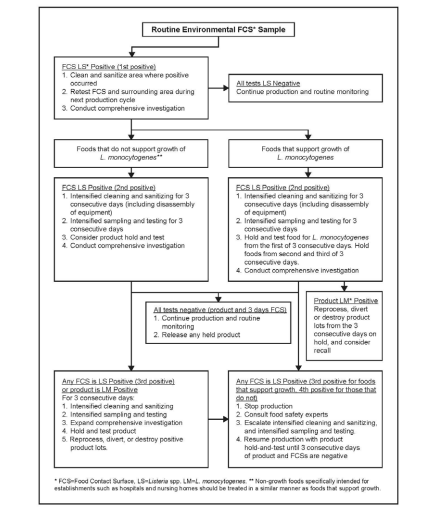
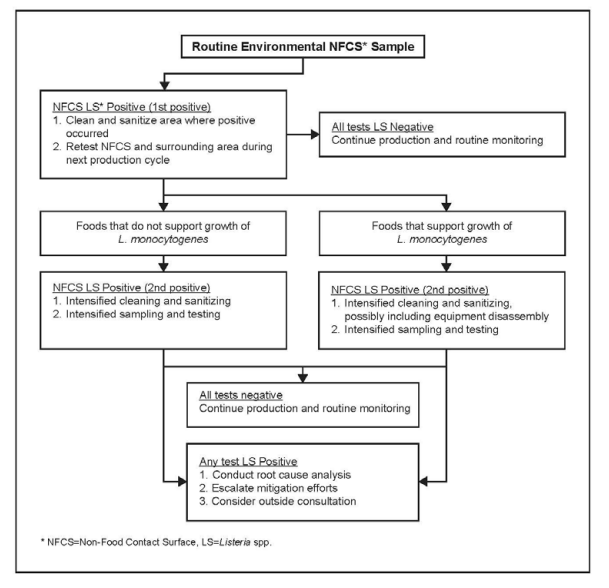
⇒ How can corrective actions be validated?
- Implement a “test and hold” policy to ensure safety before product release.
- Increase sampling frequency in previously affected areas.
- Conduct staff retraining on sanitation and hygiene practices.
Advancing EMP Effectiveness Over Time for Continuous Improvement
⇒ How should EMP data be managed and utilized?
- Digital Recordkeeping: Transitioning from manual logs to software-based tracking improves data accessibility and accuracy.
- Trend Analysis: Identifying patterns in contamination helps predict and prevent future incidents.
- Predictive Risk Assessment: Using historical data enhances proactive decision-making.
⇒ How can an EMP be continuously enhanced?
- Periodic Review & Updates: Align with evolving food safety standards and scientific advancements.
- Technology Integration: AI-powered analytics and predictive modeling can enhance risk assessments.
- Ongoing Training & Knowledge Sharing: Staying informed about industry trends and best practices fosters continuous improvement.
A well-implemented EMP isn’t just about ticking regulatory boxes—it’s a proactive shield protecting both your products and consumers. By setting up a strong monitoring framework, analyzing data effectively, and responding quickly to potential threats, you’re investing in the long-term safety and success of your business.
Ready to elevate your EMP strategy? Start with a robust environmental monitoring strategy in a tech-backed manner with Smart EMP!
Set up Your Environmental Monitoring Program the Right Way With Smart EMP
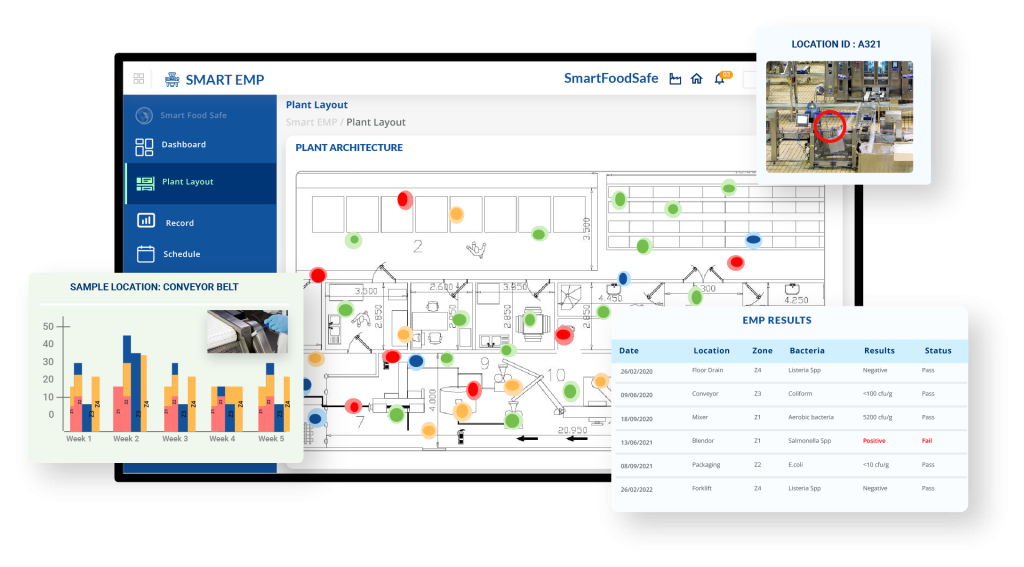
Traditional Environmental Monitoring Programs (EMPs) can be time-consuming, reactive, and prone to inefficiencies. But what if your EMP could work smarter, not harder? Smart EMP can assist you in doing just that!
Smart EMP revolutionizes environmental monitoring by automating processes from end to end, ensuring accurate assessments of plant hygiene and compliance. With built-in FDA Listeria guidelines, businesses can align their monitoring efforts with regulatory requirements seamlessly. The software offers a digital plant layout with drag-and-drop identifiers for swabbing zones, equipment, and drains, providing a visual representation of potential contamination hotspots. Configurable sampling zones, automated corrective actions, and vector swabbing enhance the precision of environmental risk management.
The platform integrates both fixed and random sampling schedules, allowing for systematic yet flexible monitoring. Internal and third-party laboratory processes are streamlined, from generating chain-of-custody forms to performing in-house analysis with effortless result updates. A robust dashboard provides real-time insights through trend analysis, heat maps, and scheduled reports, offering a data-driven approach to food safety. By digitalizing environmental control and compliance verification, Smart EMP simplifies risk detection and ensures a proactive, well-regulated production environment.