Listeria remains a lingering threat in food manufacturing, notorious for its persistence and ability to evade standard control measures. The presence of this resilient foodborne pathogen in ready-to-eat (RTE) food production poses serious public health risks, requiring specific measures to prevent outbreaks.
Food business operators (FBOs) play a critical role in mitigating this risk by implementing a targeted Environmental Monitoring Program (EMP) tailored to their facility’s needs. A well-structured EMP encompasses sampling, analysis, data interpretation, and corrective actions, ensuring early detection and control of Listeria contamination. But importantly, it should be understood that EMPs should complement—not replace—end-product testing to provide a comprehensive food safety approach.
An effective Listeria management plan incorporating a diligent and data-driven EMP designed using a risk-based strategy within the regulatory system requires strong leadership and collective commitment from all personnel to reinforce food safety compliance throughout the processing environment.
Understanding Ready-to-Eat Foods & Their Listeria Risk Profile
RTE foods include a broad category of products that do not require further preparation, such as washed and cut fruits and vegetables, deli meats, dairy products like soft cheeses, and pre-packaged salads. Outbreaks linked to Listeria in RTE foods have been widely reported, emphasizing the importance of strict adherence to Listeria testing protocols and environmental controls to minimize contamination risks in RTE food production and distribution.
Factors Influencing Listeria Growth in RTE Foods
Not all RTE foods pose the same risk for Listeria growth. The potential for contamination and bacterial proliferation largely depends on the food’s physicochemical properties, including pH levels, water activity, and storage conditions. According to internationally established criteria (Codex, 2007), RTE foods that do not support Listeria growth typically fall into one of the following categories:
- Highly acidic foods (pH < 4.4), regardless of water activity
- Low moisture foods (water activity < 0.92), regardless of pH
- A combination of acidity and low moisture (pH < 5.0 and water activity < 0.94)
Furthermore, frozen RTE foods—such as ice cream or products intended to be thawed before consumption—do not support Listeria growth due to low temperatures. However, contamination can still occur during post-processing handling, making proper hygiene and storage conditions critical.
A Closer Look at the Threat: Listeria monocytogenes
Listeria monocytogenes is a formidable pathogen. It can survive and even grow under refrigeration, withstand acidic conditions, and resist common sanitizers. This resilience makes it a persistent threat in food processing environments, particularly in facilities producing RTE foods, which are consumed without further cooking. The consequences of Listeria contamination can be severe, ranging from costly product recalls to devastating outbreaks of listeriosis, a disease with a high mortality rate, especially among vulnerable populations such as the elderly, pregnant women, and immunocompromised individuals.
Given these risks, regulatory bodies worldwide have established stringent guidelines for Listeria control in RTE food plants. However, compliance alone is not enough. A proactive EMP can help you identify and mitigate risks before they escalate, safeguarding both your consumers and your business.
Establishing a Microbiological EMP for Risk-Based Environmental Testing in RTE Food Factories
The key components essential to set up a microbiological EMP will be as follows:
1. FOOD SAFETY TEAM
An effective EMP starts with a well-trained food safety team, which may vary in size depending on the facility. Senior management’s commitment is crucial for resource allocation. The team should include personnel from food safety management, operations, microbiology, and sanitation. The team should collaborate on sample types, locations, frequency, and procedures to ensure plant hygiene and regulatory compliance.
2. OBJECTIVE OF EMP
Your EMP can be built specifically to monitor the occurrence of Listeria spp. or specifically on L. monocytogenes, which is the only pathogenic species to humans with specific regulatory limits in ready-to-eat (RTE) foods. While detecting L. monocytogenes is critical due to its pathogenic nature, monitoring for other Listeria species can serve as an early warning system for potential contamination risks.
An EMP aims to verify the effectiveness of control programs, determine the prevalence and sources of Listeria in the processing environment, or evaluate corrective actions. Importantly, the EMP is not about confirming finished product compliance but about monitoring the environment prior to reducing risks to consumers of RTE foods.
3. HYGIENE AREAS OF CONTROL
To successfully manage Listeria contamination, the EMP team must identify vulnerable areas within the plant where Listeria spp. or L. monocytogenes may contaminate the product. A key strategy is segregating areas handling raw ingredients from those where finished products could be exposed to cross-contamination through physical barriers and restrictions on personnel and material movement.
Food processing areas are divided into different hygienic zones based on contamination risk:
| Zone | Description | Examples |
|---|---|---|
| Zone 1 | Highest risk: Direct food contact surfaces | Conveyor belts, slicers, filling machines, food trays |
| Zone 2 | Near food contact surfaces | Equipment frames, control panels, sides of tunnels |
| Zone 3 | Non-food contact but near production | Floors, walls, drains, forklifts, carts |
| Zone 4 | Non-food areas | Offices, locker rooms, restrooms, storage areas |
The level of risk for each area is assessed based on factors like the potential for L. monocytogenes growth in the product and the effectiveness of microbiological lethal steps. Areas are categorized into low, medium, high-risk, and non-food production areas. A color-coded map can help identify risk levels and determine appropriate hygiene and segregation measures.
4. SAMPLING LOCATIONS
After mapping hygienic control areas, sampling sites should be chosen based on the four-zone system with decreasing levels of contamination risk. High-risk areas (Zones 1 & 2) require more frequent testing. The sampling plan should rotate across different production shifts to provide a comprehensive contamination profile. EMPs should be tailored to facility-specific risks and continuously reviewed to incorporate findings from past testing.
5. SAMPLE COLLECTION TIMES
Sample collection timing depends on the objective:
- Pre-operational samples (before processing) verify cleaning effectiveness.
- In-process samples (during production) detect contamination from biofilms or operational practices.
- End-of-production samples help assess contamination build-up during production runs.
Sampling should be conducted periodically across different shifts to ensure a representative assessment.
6. SAMPLING PROCEDURES
Aseptic sampling techniques are significant in preventing cross-contamination. Trained personnel should follow strict hygiene protocols, including:
- Using sterile swabs or sponges for collection.
- Employing neutralizing diluents when sampling post-disinfection.
- Wiping the sampling area thoroughly to capture potential contamination.
- Refrigerating (not freezing) samples and analyzing them within 24 hours.
Swabs from different locations should be collected separately, though composite sampling may be used for established EMPs with low detection rates. After sampling, sites must be sanitized immediately to avoid contamination.
7. FREQUENCY & NUMBER OF SAMPLES
The sampling plan in an EMP depends on various factors such as plant size, complexity, workflow, production volume, equipment design, cleaning procedures, and past sample data. Higher-risk areas require more frequent sampling. When implementing an EMP, a baseline should be established by collecting as many samples as possible during the first few months. Once the baseline is established, the sampling frequency can be reduced and adjusted based on previous trends.
Sampling is based on the zoning concept, with a larger portion of samples collected from high-risk Zones 1 and 2 (10-20% and 40-50%, respectively), and fewer from Zones 3 and 4. Zone 4 samples are mainly collected during investigations. Consistently negative results may indicate the need to reassess sampling locations and frequency.
8. ANALYTICAL METHODS
Analytical methods for detecting Listeria must be recognized by national or international food safety authorities (e.g., U.S. FDA, CFIA, EFSA) or validated by recognized bodies like AOAC through ring trials. The choice of detection or enumeration method depends on the sampling objective and surface type. Swabs are typically used for detection (positive/negative results) but may be used for enumeration only within the same facility, as comparisons between facilities are not possible with this method.
9. RESULT REPORTING & DATA INTERPRETATION
Internal Testing
When samples are sent to an external lab for analysis, the results may need interpretation. The process of result reporting and data interpretation involves analyzing environmental and food samples for Listeria with results reported as positive/negative or in CFU per gram for food samples tested without enrichment. Presence of Listeria spp. indicates potential hygiene issues or biofilm, while L. monocytogenes presence may require corrective actions or a recall in regulated regions.
10. CORRECTIVE ACTIONS TAKEN IN RESPONSE TO LISTERIA DETECTION
When Listeria is detected in the environment or on food contact surfaces, a tailored corrective action plan must be initiated promptly to identify the contamination source and minimize cross-contamination risks. Corrective actions should be adapted to the situation, considering the type of contamination (food contact, non-food contact, or food), the type of food, the microorganism present, and the frequency of positive results.
- Investigational Sampling: Intensified sampling of surrounding areas and sites helps determine if contamination is transient or persistent, guiding corrective actions.
- Cleaning and Disinfection: Thorough cleaning and disinfection are needed after a positive result, with follow-up testing to ensure eradication and enhanced procedures if contamination persists.
- Review of Workflow Patterns: Evaluating the movement of people and products helps prevent contamination spread and organizes workflows.
- Staff Training: Ongoing training on food safety procedures ensures staff understand their role in controlling Listeria and maintaining hygiene.
- Mapping and Trend Analysis: Regular mapping and trend analysis help identify contamination hotspots for targeted corrective measures.
- Resampling: Resampling after corrective actions verifies that contamination is controlled before resuming production.
These corrective actions, while essential for controlling Listeria, may not entirely eliminate the problem due to the organism’s ubiquitous nature. Continuous monitoring, maintenance of hygiene standards, and diligent adherence to the corrective action plan are necessary to manage the risk of Listeria contamination effectively.
Get Rid of Listeria Risks From Your RTE Food Facility with Smart EMP
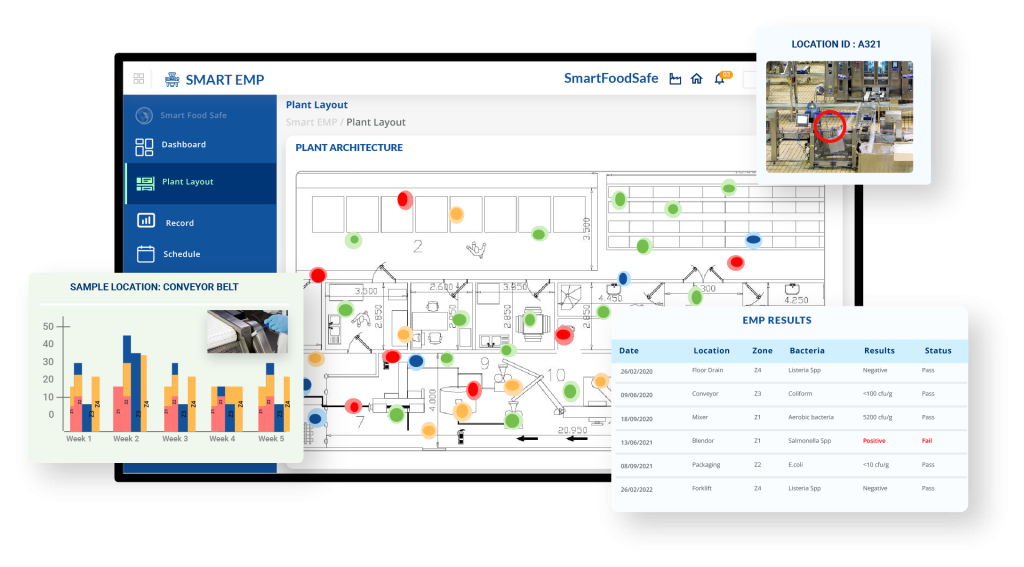
For decades, traditional environmental testing relied on manual sampling and data tracking, which can be time-consuming, error-prone, and ineffective in catching early warning signs. To stay ahead of today’s Listeria risks, food facilities need an innovative, data-driven approach that goes beyond reactive testing. This is how Smart EMP came into existence – a software solution designed to streamline environmental monitoring, automate compliance, and provide real-time insights for thorough Listeria detection and control. Smart EMP’s following features can help you implement a digital EMP at your establishment:
Automated EMP Workflow: Digitally schedule and track environmental monitoring activities, ensuring consistent sampling and compliance verification.
Built-in FDA Listeria Guidelines: Align inspections with FDA regulations using integrated guidelines to meet regulatory requirements effortlessly.
Digital Plant Layout & Visualization: Utilize a digital floor plan with swabbing zones, heat maps, and harborage maps to identify contamination hotspots effectively.
Configurable Sampling & Testing: Define sampling zones, set test parameters, and assign markers for drains, equipment, and high-risk areas.
Automated Corrective Actions: Trigger predefined corrective actions for non-compliance, including vector swabbing and scheduling re-tests.
Compliance Scheduling & Monitoring: Implement fixed, random, or hybrid scheduling to ensure periodic and risk-based sampling coverage.
Integration with Laboratory Processes: Seamlessly coordinate in-house and third-party lab analyses with digital documentation and automated result uploads.
Comprehensive Reporting & Trend Analysis: Generate heat maps, dashboards, and reports on demand to track contamination trends and take data-driven preventive actions.