On June 11th, 2024, the FDA announced a recall of individually wrapped sandwiches from an Ohio-based food wholesaler after environmental samples tested positive for Listeria monocytogenes. This incident underscores the critical need for proactive risk-based environmental monitoring programs (EMP) in food production.
With approximately 1,600 cases of listeriosis and 260 deaths annually in the U.S., as per CDC statistics, such programs could prevent contamination and subsequent recalls. The food sector must prioritize comprehending and controlling Listeria risks through continuous monitoring of potential contamination points to safeguard public health.
Understanding Listeria & Its Risks
Reference: https://www.poison.org/articles/what-is-listeria
Listeria is a genus of Gram-positive bacteria, with Listeria monocytogenes being the primary pathogenic species responsible for listeriosis, a severe foodborne illness. This rod-shaped, motile bacterium is notably persistent in food manufacturing settings. Listeriosis has a high mortality rate, particularly in cases of listerial meningitis, septicemia, and perinatal/neonatal infections, with the overall case-fatality rate ranging from 15% to 30%. Symptoms range from mild, flu-like symptoms in healthy individuals to severe neurological issues in vulnerable populations.
Listeria is commonly found in soil, water, vegetation, feed, industrial plants, and farms, and can be easily isolated from humans, domestic animals, raw agricultural and fishery products, food processing environments, and homes. This demonstrates the high prevalence of Listeria in the environment, which can be controlled by an effective environmental monitoring program.
Risk-Based Environmental Monitoring Programs (EMP) & Importance of Sampling
Environmental Monitoring Programs (EMPs) are integral in ensuring product safety and regulatory compliance for food manufacturers. They involve the systematic sampling and testing of the production environment for potential sources of contamination.
A risk-based EMP is a strategic approach designed to manage environmental risks by prioritizing resources and actions based on the likelihood and impact of potential hazards, focusing on areas where the risk of contamination is highest, to safeguarding product integrity and public health.
Sampling is a cornerstone of a risk-based EMP, as it provides the empirical data necessary to assess and monitor environmental conditions. By analyzing samples from various stages of the production process, companies can identify trends, pinpoint contamination sources, and implement corrective actions before issues escalate into larger problems.
How Risk-Based Environmental Sampling Can Curb the Prevalence of Listeria?
A well-designed risk-based Listeria EMP ensures consumer protection and regulatory compliance by identifying probable entry, harboring, and movement points of Listeria within the facility, which is the first step in preventing contamination of production surfaces and finished products. This process is often referred to as “Seek and Destroy”—a method pioneered by John Butts, Ph.D.—with the “seek” component being the monitoring program.
⇒ Risk Assessment
Risk assessment is a systematic process used to identify, evaluate, and estimate the levels of risk associated with specific hazards. In the context of controlling Listeria in food processing, it involves:
- Hazard Identification: Determining potential sources of Listeria contamination.
- Risk Analysis: Evaluating the likelihood and severity of contamination events.
- Risk Evaluation: Comparing the estimated risks against predetermined safety criteria.
⇒ Risk-Based Sampling Program
Traditional random sampling methods may not be sufficient to detect Listeria in complex food processing environments. Risk-based sampling, however, targets specific areas and processes where the risk of contamination is highest, improving the likelihood of detecting Listeria early and enabling better measures against contamination.
⇒ Building a Risk-Based Environmental Sampling Program
FDA advises using a risk-based approach for environmental monitoring procedures, establishing strategies based on food product characteristics and processing methods, with increased sampling, testing, and stringent corrective actions for higher-risk foods susceptible to Listeria contamination and growth.
Below is a quick look at how to build a risk-based environmental sampling program, as per the FDA’s Guidance for Industry to Control Listeria:
-
Mapping the Facility
Start by creating a detailed map of the processing facility, identifying all areas where food is handled, processed, and stored.
-
Identifying High-Risk Areas
Based on the risk assessment, pinpoint areas that are prone to Listeria contamination. The FDA suggests categorizing areas in the plant based on their potential for product contamination to facilitate the collection and testing of environmental samples for Listeria. One effective method is to use a zone system to characterize the plant.
-
Developing a Sampling Plan
A sampling plan must be developed based on a thorough risk assessment, considering factors such as the history of contamination, the nature of the products, and regulatory requirements. The plan should specify the locations, frequency, and methods of sample collection. The data obtained from these analyses are crucial for identifying deviations from acceptable environmental standards and for making informed decisions about necessary interventions.
A well-structured environmental sampling program with a “Seek and Destroy” approach methodically determines what, how, where, and when to sample.
What to Sample
- The most important aspect of what to sample is to identify whether you are testing for Listeria spp. or L. monocytogenes.
- The FDA recommends that you test for Listeria spp because doing so will detect both L. monocytogenes as well as species of Listeria that are more common than L. monocytogenes and allow you to correct situations that could potentially lead to contamination with L. monocytogenes.
- List of Potential Sampling Sites: Environmental monitoring procedures should specify an appropriate number of sampling sites, with an extensive list of potential sites from which some are randomly selected each time. The FDA recommends testing all sites on the list within a defined period, such as one month, and including both food-contact surfaces (FCS) and non-FCS sites in each sampling.
- High Traffic or Hard-to-Clean Environmental Surfaces: More samples should be collected from zones 1 and 2 due to higher contamination risk.
- If consistent negative test results occur, revise the procedures to add or substitute other surfaces to ensure no contamination sources are missed.
- The most important time to collect environmental samples is at a time that is several hours into production (e.g., 3 to 4 hours) or preferably just prior to cleanup, because this allows time for Listeria (if present) to work its way out of harborage sites and contaminate the environment, the processing line (including FCS sites), and, potentially, the food product.
- Samples taken too soon after sanitization may yield inaccurate results as the sanitizer may not be adequately neutralized and could interfere with the analytical test.
- Routine sampling helps in regularly monitoring and verifying sanitation and control measures’ effectiveness. The frequency of routine sampling should be risk-based, with the FDA recommending monthly sampling for low-risk foods and weekly sampling for high-risk foods. Sampling frequency should increase if Listeria spp. is detected in the plant.
- Corrective sampling investigates and confirms the source of contamination after a positive result or event. It is conducted as needed, in response to specific triggers and targets areas of concern identified during routine sampling or contamination events.
- Sampling Methods: The two most common methods for collecting samples, according to FDA are “surface sponging” and “swabbing,” with surface sponging recommended for most surfaces and swabbing for small or hard-to-access areas. Liquid rinse samples can be used for difficult-to-clean areas. Environmental sampling and testing procedures should follow authoritative references such as FDA’s BAM, ICMSF, or APHA.
- Sample Size: Sample sizes should be as large as practical, except for small nooks and crannies, and collected samples should be properly packaged with ice packs and shipped under refrigerated conditions within 24 hours, avoiding freezing.
- Analytical Techniques: The FDA recommends using their “Testing Methodology for Listeria species or L. monocytogenes in Environmental Samples” for sample analysis.
- You can test individual samples or composite samples but do not composite more than five samples to avoid reducing detection sensitivity.
- During production, samples should be collected at least 3 to 4 hours into production, and compositing is not recommended when locating the source of contamination.
- Samples can be analyzed in-house or by an external lab, ensuring the lab uses current scientifically valid methods.
Data Collection and Analysis
The FDA recommends analyzing environmental sampling data over time to identify trends, which can help improve sanitation by reducing positive environmental samples in the plant. Trend analysis can reveal uncontrolled Listeria, prompting necessary control measures. Hence, regularly collect and analyze data from the sampling program to identify trends, assess the effectiveness of control measures, and make necessary adjustments to the risk-based sampling plan.
If positive samples persist in a particular area, a more thorough investigation may be needed to identify issues such as unidentified harborage sites. Increased Listeria incidence should trigger an investigation and corrective actions to address the underlying causes.
Training Personnel
The FDA mandates that all individuals involved in food manufacturing, processing, packing, or holding receive training in food hygiene and safety, emphasizing personal health and hygiene relevant to their duties.
Training should also cover health and hygienic practices specific to controlling Listeria for all personnel and contractors in production and storage areas. This training should highlight each individual’s role in Listeria control, its importance, and management’s expectations, and be conducted before job activities begin, with annual refreshers.
Zone 1: Food-contact surfaces
Areas that come into direct contact with food, such as utensils, table surfaces, slicers, tank interiors, packaging, conveyors, etc.
Zone 2: Non-food-contact surfaces in close proximity to food and food-contact surfaces
Areas near food and food contact surfaces but do not directly contact food, such as equipment housing or framework, walls, floors, and drains near FCSs carts.
Zone 3: More remote non-food-contact surfaces
Areas that are further from the processing areas could still lead to contamination of Zones 1 and 2, such as forklifts, hand trucks, carts moving within the plant, etc.
Zone 4: Non-food-contact surfaces in remote areas
Remote areas outside the processing area where environmental pathogens can enter the processing environment, such as locker rooms, cafeterias, and areas where raw materials or finished foods are stored or transported.
Where to Sample
When to Sample
How to Sample
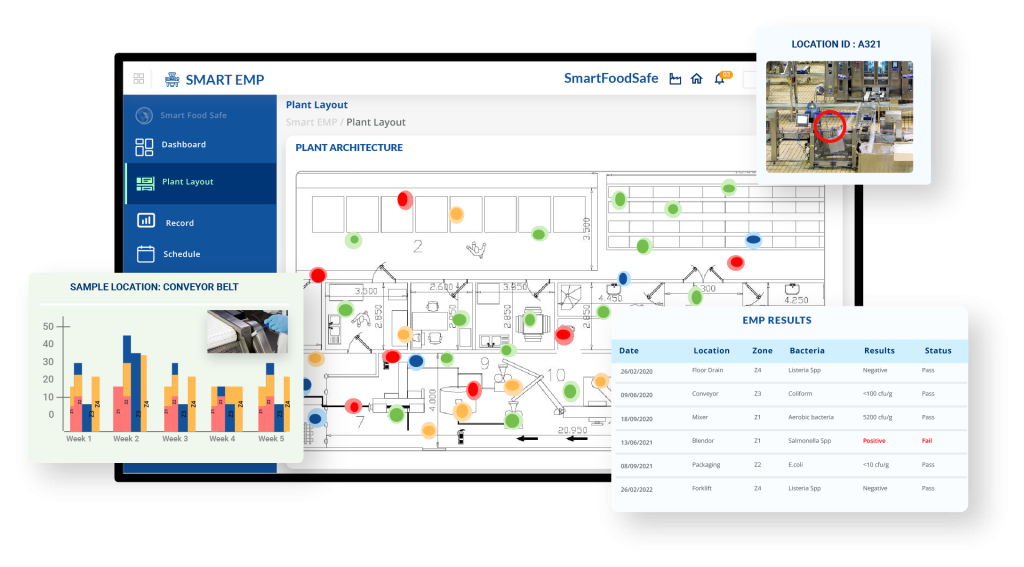
Smart EMP: Digitally Establish Efficient Risk-Based Environmental Sampling Plans to Control Listeria
Smart EMP assists food businesses with a tech-enabled solution to risk-based environmental sampling with digital features facilitating regular and systematic “Seek and Destroy” strategies for the detection and elimination of Listeria harborage sites.
This platform empowers companies to pinpoint Listeria contamination sources through consistent monitoring practices and targeted sampling of high-risk areas, tracking sanitation efficacy, and establishing a defense mechanism against Listeria, avoiding costly recalls. Through its integrated functionalities, Smart EMP ultimately strives to usher in a safer food supply chain and a proactive stance against Listeria contamination.